- 1. Why Traditional Device Management Fails
- 2. Unifying Your Fleet: A New Strategy for Medical Device Management
- 3. Compliance and Security: A Non-Negotiable Foundation
- 4. The Solution: How AirDroid Business Empowers Medical Device Management
- 5. Case Study: A Technology Company Transforms Operations with AirDroid Business
The modern healthcare landscape is defined by its reliance on smart, connected medical devices—from bedside monitors and infusion pumps to wearable sensors. Yet, this rapid technological growth has introduced a new set of challenges. For many healthcare providers and medical device manufacturers, managing this complex ecosystem is no longer a simple task of maintenance. It's a strategic imperative. Effectively managing the medical device ecosystem affects patient care, operational costs, and regulatory compliance for healthcare providers.
This article will guide you through the transition from traditional, reactive maintenance to a proactive, integrated management system. We'll explore how modern solutions, particularly through the use of powerful MDM software like AirDroid Business, can solve your biggest pain points and build a more efficient, compliant, and secure operation.
Part 1: Why Traditional Device Management Fails
Medical device management is no longer a matter of simply repairing equipment when it breaks. The traditional, siloed approach creates a host of problems:
- High On-Site Maintenance Costs: When devices are scattered across different clinics or nursing homes, sending a technician for every small issue is inefficient and expensive. Travel time, labor costs, and lost productivity quickly add up.
- A "Black Box" of Information: Without a centralized system, it's impossible to get a real-time view of device status. You can't track device health, battery levels, or usage patterns, which leads to slow responses and reactive troubleshooting.
- Inconsistent Updates: Manually updating software or configurations on a large fleet of devices is time-consuming and prone to errors. This can lead to security vulnerabilities and non-compliance.
This fragmented system creates significant risks, leading to maintenance delays, compliance gaps, and wasted resources.
Part 2: Unifying Your Fleet: A New Strategy for Medical Device Management
The solution lies in moving from a reactive to a proactive management model. A modern approach to medical device management hinges on a centralized, unified platform that integrates data and operations across your organization.
This strategy focuses on:
- Centralized Control: Consolidating all device information, from procurement details to maintenance history, into a single dashboard. This breaks down departmental silos and ensures everyone is working with the same data.
- Predictive Maintenance: Using real-time data and analytics to anticipate device failures before they happen. Instead of waiting for a device to break, you can schedule maintenance proactively, minimizing downtime and costs.
- Data-Driven Decisions: Leveraging device data to inform strategic decisions. This includes optimizing device procurement, managing asset allocation, and justifying maintenance budgets with hard numbers.
This holistic approach transforms device management from a burdensome task into a powerful tool for operational excellence.
Part 3: Compliance and Security: A Non-Negotiable Foundation
For any medical device management system to be successful, compliance and security must be its core pillars. This is not just about avoiding fines—it's about protecting patient data and ensuring safe care.
- Quality Management Systems (QMS): Modern solutions must support compliance with industry standards like ISO 13485 and FDA 21 CFR Part 820. A robust system helps you maintain meticulous records, standardize procedures, and ensure every device meets stringent quality requirements.
- Risk Management: A good management system embeds risk assessment into every stage of the device lifecycle. It helps you identify, analyze, and mitigate potential risks to both device performance and patient safety.
- Data Security: With devices handling sensitive patient information, robust security is paramount. Your system must offer features like data encryption, granular user access controls, and comprehensive audit trails to comply with regulations such as HIPAA and GDPR.
These elements are essential for building trust and maintaining your reputation in a highly regulated industry.
Part 4: The Solution: How AirDroid Business Empowers Medical Device Management
To effectively address the challenges of modern medical device management, you need a powerful and flexible MDM solution. AirDroid Business is designed to provide the specific tools and features necessary to manage a distributed fleet of medical devices, ensuring security, efficiency, and compliance.
Here's how AirDroid Business delivers precise empowerment for medical devices:
- Unrivaled Remote Control & Troubleshooting: With AirDroid Business, your technical staff can remotely access and control any device, as if they were holding it in their hands. This eliminates the need for expensive on-site visits. Technicians can view device logs, restart a device, or adjust settings from a central location, drastically reducing downtime and operational costs.
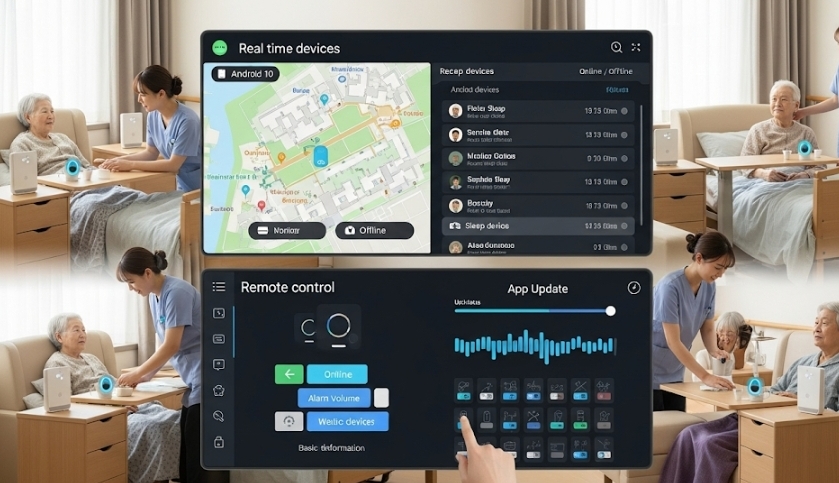
- The Device Wall: A Centralized Command Center: Our "Device Wall" feature offers a real-time, visual overview of your entire device fleet. You can instantly monitor the online status, battery level, network connectivity, and location of every device. When a device goes offline or a critical alert is triggered, your team is notified immediately, enabling a proactive response.
- Application Management Service (AMS): Effortless Bulk Updates: The AirDroid Business AMS feature streamlines the process of managing your in-house applications. You can upload your custom-built medical app—such as your sleep data collection application—to the platform and push it out to hundreds of devices simultaneously. This ensures all devices are running the latest, most secure version of the software, and it can even enforce silent installations and updates.
- Kiosk Mode for Dedicated Use: To prevent unauthorized use and maintain compliance, AirDroid Business offers a powerful Kiosk Mode. This feature locks down devices to run only a specific application or a predefined set of apps. In a nursing home, this ensures the device is used exclusively for its intended purpose (e.g., sleep monitoring) and not for other personal use.
Meeting Compliance: AirDroid Business’s Commitment to Security
Beyond core management features, AirDroid Business is built to help you meet strict industry regulations. We help you stay compliant with standards like HIPAA and GDPR by securing your device management processes. We ensure your data is protected through robust encryption and clear privacy policies. For international standards like ISO/IEC 27001 and SOC-2, we adhere to strict security controls using secure data centers and continuous monitoring. Additionally, we partner with PCI-compliant vendors for payment processing, safeguarding your transactions. This commitment to security and compliance lets you focus on patient care, knowing your devices are managed securely and responsibly.
Part 5: Case Study: A Technology Company Transforms Operations with AirDroid Business
A technology company specializing in smart devices for elderly care develops and sells sleep monitoring equipment. These devices, running on Android 10, are deployed in nursing homes to collect sleep data, helping caregivers monitor residents' sleep quality and duration.
The Challenge
The company faced significant operational challenges with its dispersed device fleet:
- 1. High On-Site Maintenance Costs: Sending technicians to various locations for every device issue was expensive and inefficient.
- 2. Lack of Centralized Monitoring: It was impossible to monitor the status of all devices in real-time, leading to slow response times for failures.
- 3. Risk of Misuse: The devices were intended for sleep monitoring only, but without control, they were susceptible to unauthorized use.
- 4. Inefficient App Updates: Manually updating their proprietary app on a large number of devices was time-consuming and prone to errors.
AirDroid Business Solution
- 1. The company implemented AirDroid Business to address these pain points with a comprehensive MDM solution:
- 2. Remote Control: Technicians could remotely access and troubleshoot devices instantly, eliminating the need for costly on-site visits.
- 3. Device Wall: A centralized dashboard provided a real-time overview of every device's status, including online presence, battery level, and location, enabling proactive monitoring.
- 4. Application Management Service (AMS): This feature allowed the company to push updates for their proprietary app to all devices at once, ensuring consistency and security.
- 5. Kiosk Mode: Devices were locked down to run only the specific sleep monitoring app, preventing misuse and ensuring the devices were used for their intended purpose.
The Results
By adopting AirDroid Business, the company transformed its operational model from reactive to proactive. They dramatically reduced maintenance costs, improved troubleshooting efficiency, and secured their devices, ultimately enhancing the reliability of their service for caregivers and residents.
Conclusion: Building a Future-Proof Medical Device Management Ecosystem
Effective medical device management is the foundation of a safe, efficient, and compliant healthcare operation. By moving away from outdated, manual processes and embracing a modern MDM solution like AirDroid Business, you can address the core challenges of today's complex device ecosystem.
Implementing a robust management platform is more than a technical upgrade; it's a strategic investment in reducing costs, enhancing patient safety, and future-proofing your organization. Take the first step toward a more integrated and intelligent management system today.





Leave a Reply.